CSL Vifor and Travere Therapeutics’ FILSPARI (sparsentan) Received European Commission Approval Against IgA Nephropathy
Shots:
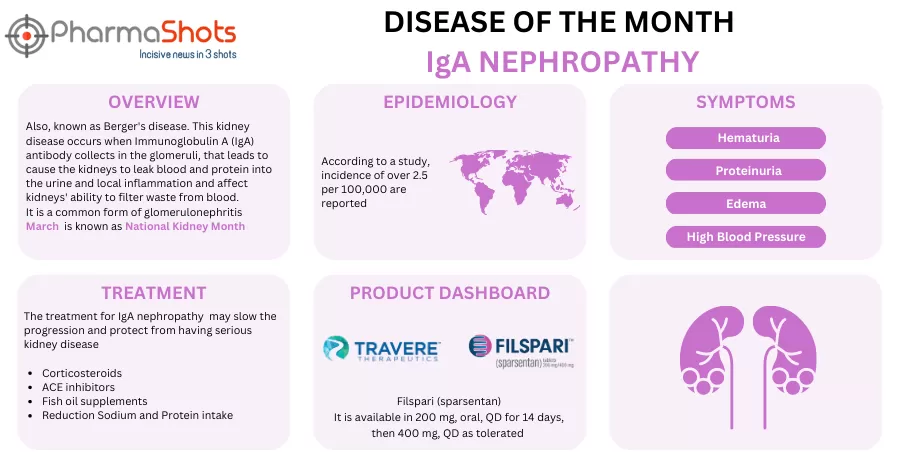
- FILSPARI received conditional marketing authorization (CMA) from European Commission for treatment of adults with primary IgAN with a urine protein excretion ≥1.0 g/day (Urine Protein: Creatinine > 0.75g/g)
- The approval is based on PROTECT (P-III) study, evaluating safety and efficacy of sparsentan (400 mg) vs irbesartan (300 mg). Study met 1EPs: statistical significance at pre specified interim analysis; mean reduction in proteinuria with Sparsentan (49.8%) and Irbesartan (15.1%) after 36wks
- First EU market launch of FILSPARI, a non-immunosuppressive therapy is expected in H2’24
Ref: CSL Vifor | Image: CSL Vifor
Related News:- CSL Vifor and Travere Therapeutics’ Filspari (sparsentan) Gains CHMP’s Positive Opinion to Treat IgA Nephropathy
PharmaShots! Your go-to media platform for customized news ranging for multiple indications. For more information connect with us at connect@pharmashots.com
Click here to read the full press release
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.